PhD student
Email

Carlos Ibanez Lab @ PKU & CIBR
McGovern Institute at Peking University & Chinese Institute for Brain Research
PhD student
Email

Administrative Assistant
Email

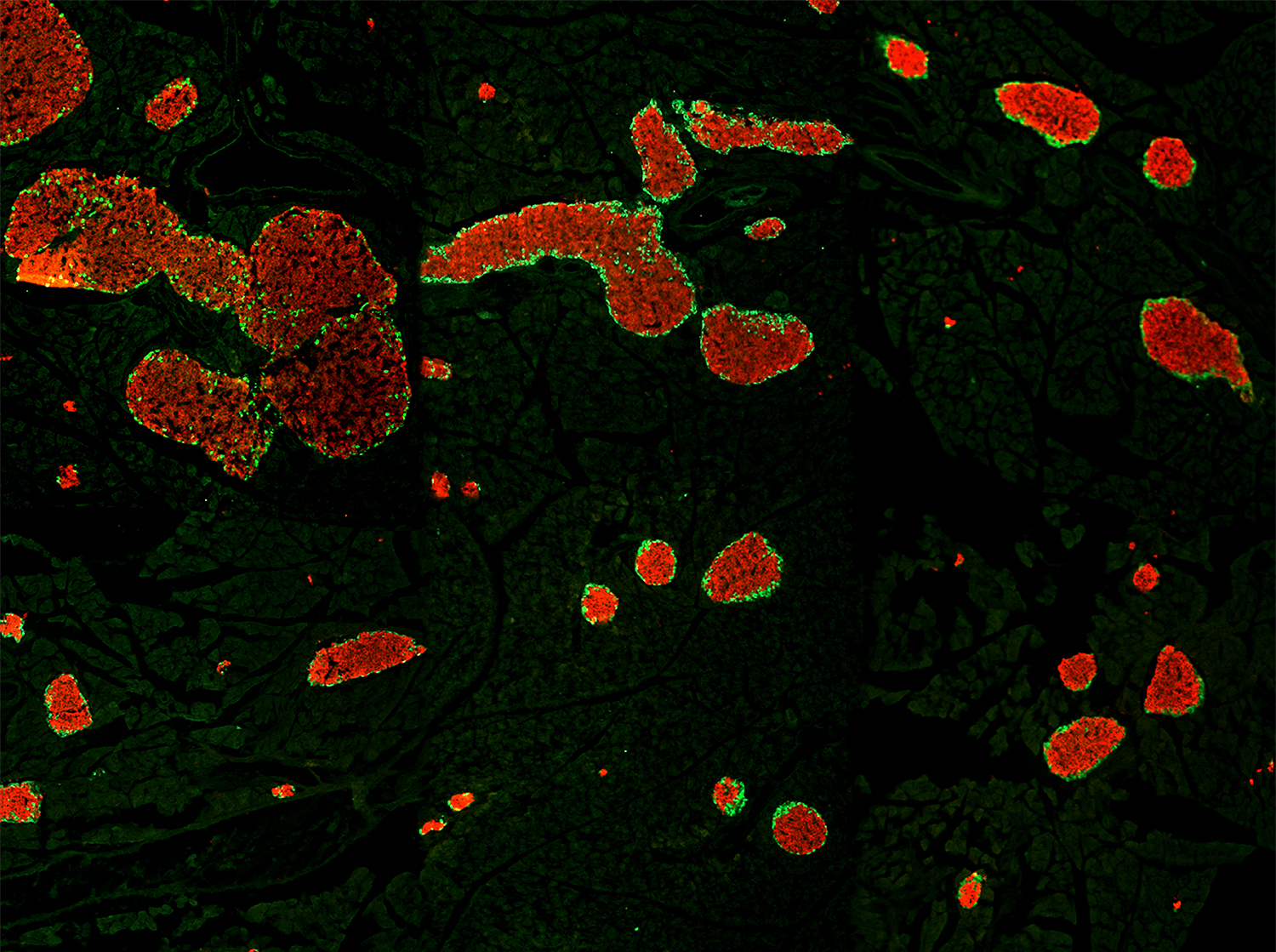
The medial habenula (mHb) is an understudied small brain nucleus linking forebrain and midbrain structures controlling anxiety and fear behaviors. The mechanisms that maintain the structural and functional integrity of mHb neurons and their synapses remain unknown.
In this study, we used spatiotemporally controlled Cre-mediated recombination in adult mice, and found that the glial cell–derived neurotrophic factor receptor alpha 1 (GFRα1) is required in adult mHb neurons for synaptic stability and function. mHb neurons express some of the highest levels of GFRα1 in the mouse brain, and acute ablation of GFRα1 results in loss of septo-habenular and habenulo-interpeduncular glutamatergic synapses, with the remaining synapses displaying reduced numbers of presynaptic vesicles. Chemo- and optogenetic studies in mice lacking GFRα1 revealed impaired circuit connectivity, reduced AMPA receptor postsynaptic currents, and abnormally low rectification index of AMPARs, suggesting reduced Ca2+ permeability. Further biochemical and proximity ligation assay studies defined the presence of GluA1/GluA2 (Ca2+ impermeable) as well as GluA1/GluA4 (Ca2+ permeable) AMPAR complexes in mHb neurons, as well as clear differences in the levels and association of AMPAR subunits with mHb neurons lacking GFRα1. Finally, acute loss of GFRα1 in adult mHb neurons reduced anxiety-like behavior and potentiated context-based fear responses, phenocopying the effects of lesions to septal projections to the mHb.
These results uncover an unexpected function for GFRα1 in the maintenance and function of adult glutamatergic synapses and reveal a potential new mechanism for regulating synaptic plasticity in the septo-habenulo-interpeduncular pathway and attuning of anxiety and fear behaviors.
The paper has been published in PLOS Biology
Read the full paper HERE

Adult medial habenula neurons require GDNF receptor GFRα1 for synaptic stability and function
Diana Fernández-Suárez , Favio A. Krapacher, Katarzyna Pietrajtis, Annika Andersson, Lilian Kisiswa, Alvaro Carrier-Ruiz, Marco A. Diana and Carlos F. Ibáñez (2021)
PLOS Biology (2021), vol 19(11): e3001350
Click on thumbnail to display PDF
PhD student
Email

Sustained anti-obesity effects of life-style change and anti-inflammatory interventions after conditional inactivation of the activin receptor ALK7
Rajkamal Srivastava, Ee-Soo Lee, Eunice Sim, New Chih Shen and Carlos F. Ibáñez (2021)
The FASEB Journal 2021;35:e21759
Click on thumbnail to display PDF
Life- style change and anti-inflammatory interventions have only transient effects in obesity. It is not clear how benefits obtained by these treatments can be maintained longer term, especially during sustained high caloric intake. Constitutive ablation of the activin receptor ALK7 in adipose tissue enhances catecholamine signaling and lipolysis in adipocytes, and protects mice from diet-induced obesity.
In this study, we investigated the consequences of conditional ALK7 ablation in adipocytes of adult mice with pre- existing obesity. Although ALK7 deletion had little effect on its own, it synergized strongly with a transient switch to low- fat diet (life-style change) or anti-inflammatory treatment (Na-salicylate), resulting in enhanced lipolysis, increased energy expenditure, and reduced adipose tissue mass and body weight gain, even under sustained high caloric intake. By themselves, diet- switch and salicylate had only a temporary effect on weight gain. Mechanistically, combination of ALK7 ablation with either treatment strongly enhanced the levels of β3-AR, the main adrenergic receptor for catecholamine stimulation of lipolysis, and C/EBPα, an upstream regulator of β3-AR expression. These results suggest that inhibition of ALK7 can be combined with simple interventions to produce longer- lasting benefits in obesity.
The paper has been published in The FASEB Journal.
Read the full paper HERE
Regulation of metabolic homeostasis by the TGF-b superfamily receptor ALK7
Ibanez, C.F (2021
FEBS Journal, June 2021, doi:10.1111/febs.16090
Click on thumbnail to display PDF
PhD student
Email
