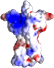
After quite some perseverance and few years of work, the 3D structure of the N-terminal portion of the extracellular domain (ECD) of the RET receptor has been determined by former postdoc fellow Svend Kjaer working at the lab of Nel McDonald in CRUK, London. The paper has appeared online in Nature Structure and Molecular Biology. During his tenure at our lab in 1998-2004, Svend studied RET intensively and published several papers on its biosynthesis, oligomerization and structure-function relationships. He spent several years producing fragments of the RET extracellular domain for structural characterization. Although sufficient for biochemical analyses, the yield was never enough for crystallization. The breakthrough at CRUK came after introduction of a few mutations, including two that knocked-off a couple of unpaired cysteines. The new structure gives tantalizing insights into how RET interacts with the GDNF ligand and co-receptor GFRa1 and how its mutation causes disease.
|
|||||
Former postdoc fellow Svend Kjaer gets first glimpse at the structure of the Ret ECD |
|||||
|
Copyright © 2025 Carlos Ibanez Lab @ KI - All Rights Reserved Powered by WordPress & Atahualpa |
|||||


Leave a Reply