|
|
Genome-wide studies have identified three missense variants in the human gene ACVR1C, encoding the TGF-β superfamily receptor ALK7, that correlate with altered waist-to-hip ratio adjusted for body mass index (WHR/BMI), a measure of body fat distribution.
In our latest paper, to move from correlation to causation and understand the effects of these variants on fat accumulation and adipose tissue function, we introduced each of the variants in the mouse Acvr1c locus and investigated metabolic phenotypes in comparison with a null mutation.
Mice carrying the I195T variant showed resistance to high fat diet (HFD)-induced obesity, increased catecholamine-induced adipose tissue lipolysis and impaired ALK7 signaling, phenocopying the null mutants. Mice with the I482V variant displayed an intermediate phenotype, with partial resistance to HFD-induced obesity, reduction in subcutaneous, but not visceral, fat mass, decreased systemic lipolysis and reduced ALK7 signaling. Surprisingly, mice carrying the N150H variant were metabolically indistinguishable from wild type under HFD, although ALK7 signaling was reduced at low ligand concentrations.
Together, these results validate ALK7 as an attractive drug target in human obesity and suggest a lower threshold for ALK7 function in humans compared to mice.
The paper has been published in Molecular Metabolism
How receptors juggle their interactions with multiple downstream effectors remains poorly understood.
In our latest paper, we report that the outcome of death receptor p75NTR signaling is determined through competition of effectors for interaction with its intracellular domain, in turn dictated by the nature of the ligand. While NGF induces release of RhoGDI through recruitment of RIP2, thus decreasing RhoA activity in favor of NFkB signaling, MAG induces PKC-mediated phosphorylation of the RhoGDI N-terminus, promoting its interaction with the juxtamembrane domain of p75NTR, disengaging RIP2, and enhancing RhoA activity in detriment of NF-kB. This results in stunted neurite outgrowth and apoptosis in cerebellar granule neurons. If presented simultaneously, MAG prevails over NGF. The NMR solution structure of the complex between the RhoGDI N-terminus and p75NTR juxtamembrane domain reveals previously unknown structures of these proteins and clarifies the mechanism of p75NTR activation.
These results show how ligand-directed competition between RIP2 and RhoGDI for p75NTR engagement determine axon growth and neuron survival. Similar principles are likely at work in other receptors engaging multiple effectors and signaling pathways.
The paper has been published in EMBO Reports
Adipocyte hyperplasia and hypertrophy are the two main processes contributing to adipose tissue expansion, yet the mechanisms that regulate and balance their involvement in obesity are incompletely understood. Activin B/GDF-3 receptor ALK7 is expressed in mature adipocytes and promotes adipocyte hypertrophy upon nutrient overload by suppressing adrenergic signaling and lipolysis. In contrast, the role of ALK4, the canonical pan-activin receptor, in adipose tissue is unknown.
In our latest paper, we report that, unlike ALK7, ALK4 is preferentially expressed in adipocyte precursors, where it suppresses differentiation, allowing proliferation and adipose tissue expansion. ALK4 expression in adipose tissue increases upon nutrient overload and positively correlates with fat depot mass and body weight, suggesting a role in adipose tissue hyperplasia during obesity. Mechanistically, ALK4 signaling suppresses expression of CEBPα and PPARγ, two master regulators of adipocyte differentiation. Conversely, ALK4 deletion enhances CEBPα/PPARγ expression and induces premature adipocyte differentiation, which can be rescued by CEBPα knockdown.
These results clarify the function of ALK4 in adipose tissue and highlight the contrasting roles of the two activin receptors in the regulation of adipocyte hyperplasia and hypertrophy during obesity.
The paper has been published in The Journal Of Biological Chemistry
ΔfosB is an alternatively spliced product of the FosB gene that is essential for dopamine-induced reward pathways and that acts as a master switch for addiction. However, the molecular mechanisms of its generation and regulation by dopamine signaling are unknown.
In this new paper, we report that dopamine D1 receptor signaling synergizes with the activin/ALK4/Smad3 pathway to potentiate the generation of ΔFosB mRNA in medium spiny neurons (MSNs) of the nucleus accumbens (NAc) via activation of the RNA-binding protein PCBP1, a regulator of mRNA splicing. Concurrent activation of PCBP1 and Smad3 by D1 and ALK4 signaling induced their interaction, nuclear translocation, and binding to sequences in exon-4 and intron-4 of FosB mRNA. Ablation of either ALK4 or PCBP1 in MSNs impaired ΔFosB mRNA induction and nuclear translocation of ΔFosB protein in response to repeated co-stimulation of D1 and ALK4 receptors. Finally, ALK4 is required in NAc MSNs of adult mice for behavioral sensitization to cocaine.
These findings uncover an unexpected mechanism for ΔFosB generation and drug-induced sensitization through convergent dopamine and ALK4 signaling.
The paper has been published in The EMBO Journal
The medial habenula (mHb) is an understudied small brain nucleus linking forebrain and midbrain structures controlling anxiety and fear behaviors. The mechanisms that maintain the structural and functional integrity of mHb neurons and their synapses remain unknown.
In this study, we used spatiotemporally controlled Cre-mediated recombination in adult mice, and found that the glial cell–derived neurotrophic factor receptor alpha 1 (GFRα1) is required in adult mHb neurons for synaptic stability and function. mHb neurons express some of the highest levels of GFRα1 in the mouse brain, and acute ablation of GFRα1 results in loss of septo-habenular and habenulo-interpeduncular glutamatergic synapses, with the remaining synapses displaying reduced numbers of presynaptic vesicles. Chemo- and optogenetic studies in mice lacking GFRα1 revealed impaired circuit connectivity, reduced AMPA receptor postsynaptic currents, and abnormally low rectification index of AMPARs, suggesting reduced Ca2+ permeability. Further biochemical and proximity ligation assay studies defined the presence of GluA1/GluA2 (Ca2+ impermeable) as well as GluA1/GluA4 (Ca2+ permeable) AMPAR complexes in mHb neurons, as well as clear differences in the levels and association of AMPAR subunits with mHb neurons lacking GFRα1. Finally, acute loss of GFRα1 in adult mHb neurons reduced anxiety-like behavior and potentiated context-based fear responses, phenocopying the effects of lesions to septal projections to the mHb.
These results uncover an unexpected function for GFRα1 in the maintenance and function of adult glutamatergic synapses and reveal a potential new mechanism for regulating synaptic plasticity in the septo-habenulo-interpeduncular pathway and attuning of anxiety and fear behaviors.
The paper has been published in PLOS Biology
Life- style change and anti-inflammatory interventions have only transient effects in obesity. It is not clear how benefits obtained by these treatments can be maintained longer term, especially during sustained high caloric intake. Constitutive ablation of the activin receptor ALK7 in adipose tissue enhances catecholamine signaling and lipolysis in adipocytes, and protects mice from diet-induced obesity.
In this study, we investigated the consequences of conditional ALK7 ablation in adipocytes of adult mice with pre- existing obesity. Although ALK7 deletion had little effect on its own, it synergized strongly with a transient switch to low- fat diet (life-style change) or anti-inflammatory treatment (Na-salicylate), resulting in enhanced lipolysis, increased energy expenditure, and reduced adipose tissue mass and body weight gain, even under sustained high caloric intake. By themselves, diet- switch and salicylate had only a temporary effect on weight gain. Mechanistically, combination of ALK7 ablation with either treatment strongly enhanced the levels of β3-AR, the main adrenergic receptor for catecholamine stimulation of lipolysis, and C/EBPα, an upstream regulator of β3-AR expression. These results suggest that inhibition of ALK7 can be combined with simple interventions to produce longer- lasting benefits in obesity.
The paper has been published in The FASEB Journal.
A prevalent model of Alzheimer’s disease (AD) pathogenesis postulates the generation of neurotoxic fragments derived from the amyloid precursor protein (APP) after its internalization to endocytic compartments. The molecular pathways that regulate APP internalization and intracellular trafficking in neurons are incompletely understood.
In this paper, we report that 5xFAD mice, an animal model of AD, expressing signaling-deficient variants of the p75 neurotrophin receptor (p75NTR) show greater neuroprotection from AD neuropathology than animals lacking this receptor. p75NTR knock-in mice lacking the death domain or transmembrane Cys259 showed lower levels of Aβ species, amyloid plaque burden, gliosis, mitochondrial stress and neurite dystrophy than global knock-outs. Strikingly, long-term synaptic plasticity and memory, which are completely disrupted in 5xFAD mice, were fully recovered in the knock-in mice. Mechanistically, we found that p75NTR interacts with APP at the plasma membrane and regulates its internalization and intracellular trafficking in hippocampal neurons. Inactive p75NTR variants internalized considerably slower than wild type p75NTR and showed increased association with the recycling pathway, thereby reducing APP internalization and colocalization with BACE1, the critical protease for generation of neurotoxic APP fragments, favoring non-amyloidogenic APP cleavage. These results reveal a novel pathway that directly and specifically regulates APP internalization, amyloidogenic processing and disease progression, and suggest that inhibitors targeting the p75NTR transmembrane domain may be an effective therapeutic strategy in AD.
The paper has been published in The EMBO Journal.
Adaptation to nutrient availability is crucial for survival. Upon nutritional stress, such as during prolonged fasting or cold exposure, organisms need to balance the feeding of tissues and the maintenance of body temperature. Mechanisms regulating the adaptation of brown adipose tissue (BAT), a key organ for non-shivering thermogenesis, to variations in nutritional state have been unknown.
In this new paper, we report that specific deletion of the activin receptor ALK7 in BAT resulted in fasting-induced hypothermia due to exaggerated catabolic activity in brown adipocytes. After overnight fasting, BAT lacking ALK7 showed increased expression of genes responsive to nutrient stress, including the upstream regulator KLF15, aminoacid catabolizing enzymes, notably proline dehydrogenase (POX), and adipose triglyceride lipase (ATGL), as well as markedly reduced lipid droplet size. In agreement with this, ligand stimulation of ALK7 suppressed POX and KLF15 expression in both mouse and human brown adipocytes. Treatment of mutant mice with the glucocorticoid receptor antagonist RU486 restored KLF15 and POX expression levels in mutant BAT, suggesting that loss of BAT ALK7 results in excessive activation of glucocorticoid signaling upon fasting. These results reveal a novel signaling pathway downstream of ALK7 which regulates the adaptation of BAT to nutrient availability by limiting nutrient stress-induced overactivation of catabolic responses in brown adipocytes
The paper has been published in eLife.
In this new paper, we report that CD137, a cell surface protein used in several studies as a marker for beige adipocytes, is undetectable at the protein level in beige adipocytes in vivo or in vitro, and its expression is not upregulated by adrenergic stimulation or cold exposure, as expected for a beige cell marker. Moreover, CD137 knock-out mice showed elevated levels of thermogenic markers, including UCP1, increased numbers of beige adipocyte precursors, and expanded UCP1-expressing cell clusters in inguinal WAT after chronic cold exposure. CD137 knock-out mice also showed enhanced cold resistance. These results indicate that CD137 functions as a negative regulator of “browning” in white adipose tissue, and call into question the use of this protein as a functional marker for beige adipocytes.
The paper has just been published in The Journal of Biological Chemistry.
In this new paper, we report how the activin receptor ALK4 coordinates signaling by activin ligands with intrinsic transcriptional programs driven by SATB1 to regulate the development of somatostatin interneurons in the developing mouse neocortex.
Although the role of transcription factors in fate specification of cortical interneurons is well established, how these interact with extracellular signals to regulate interneuron development is poorly understood. Here we show that the activin receptor ALK4 is a key regulator of the specification of somatostatin interneurons. Mice lacking ALK4 in GABAergic neurons of the medial ganglionic eminence (MGE) showed marked deficits in distinct subpopulations of somatostatin interneurons from early postnatal stages of cortical development. Specific losses were observed among distinct subtypes of somatostatin+/Reelin+ double-positive cells, including Hpse+ layer IV cells targeting parvalbumin+interneurons, leading to quantitative alterations in the inhibitory circuitry of this layer. Activin-mediated ALK4 signaling in MGE cells induced interaction of Smad2 with SATB1, a transcription factor critical for somatostatin interneuron development, and promoted SATB1 nuclear translocation and repositioning within the somatostatin gene promoter. These results indicate that intrinsic transcriptional programs interact with extracellular signals present in the environment of MGE cells to regulate cortical interneuron specification.
The paper has just been published in The Journal of Cell Biology .
Read the full paper HERE.
In this new paper, we report that abnormal TDP‐43 function culminate in impaired secretion of the neurotrophin BDNF, whose restoration is sufficient to rescue major disease phenotypes caused by aberrant TDP‐43 activity.
Aberrant function of the RNA‐binding protein TDP‐43 has been causally linked to multiple neurodegenerative diseases. Due to its large number of targets, the mechanisms through which TDP‐43 malfunction cause disease are unclear. Here, we report that knockdown, aggregation, or disease‐associated mutation of TDP‐43 all impair intracellular sorting and activity‐dependent secretion of the neurotrophin brain‐derived neurotrophic factor (BDNF) through altered splicing of the trafficking receptor Sortilin. Adult mice lacking TDP‐43 specifically in hippocampal CA1 show memory impairment and synaptic plasticity defects that can be rescued by restoring Sortilin splicing or extracellular BDNF. Human neurons derived from patient iPSCs carrying mutated TDP‐43 also show altered Sortilin splicing and reduced levels of activity‐dependent BDNF secretion, which can be restored by correcting the mutation. We propose that major disease phenotypes caused by aberrant TDP‐43 activity may be explained by the abnormal function of a handful of critical proteins, such as BDNF.
The paper has just been published in The EMBO Journal.
Read the full paper HERE.
In this new paper, we have used a novel chemical biology approach to identify a small molecule targeting the transmembrane domain of death receptor p75NTR that induces melanoma cell death and reduces tumor growth
Small molecules offer powerful ways to alter protein function. However, most proteins in the human proteome lack small-molecule probes, including the large class of non-catalytic transmembrane receptors, such as death receptors. We hypothesized that small molecules targeting the interfaces between transmembrane domains (TMDs) in receptor complexes may induce conformational changes that alter receptor function. Applying this concept in a screening assay, we identified a compound targeting the TMD of death receptor p75NTR that induced profound conformational changes and receptor activity. The compound triggered apoptotic cell death dependent on p75NTR and JNK activity in neurons and melanoma cells, and inhibited tumor growth in a melanoma mouse model. Due to their small size and crucial role in receptor activation, TMDs represent attractive targets for small-molecule manipulation of receptor function.
The paper has just been published in Cell Chemical Biology.
Read the full paper HERE.
In this new paper, we show how intracellular effectors RIP2 and TRAF6 compete for binding to the p75NTR intracellular domain to regulate cell death of cerebellar granule neurons.
Cerebellar granule neurons (CGNs) undergo programmed cell death during the first postnatal week of mouse development, coincident with sustained expression of the death receptor p75NTR. Although ablation of p75NTR did not affect CGN cell death, deletion of the downstream effector RIP2 significantly increased CGN apoptosis, resulting in reduced adult CGN number and impaired behaviors associated with cerebellar function. Remarkably, CGN death was restored to basal levels when p75NTR is deleted in RIP2-deficient mice. We found that RIP2 gates the signaling output of p75NTR by competing with TRAF6 for binding to the receptor intracellular domain. In CGNs lacking RIP2, more TRAF6 was associated with p75NTR, leading to increased JNK-dependent apoptosis. In agreement with this, pharmacological inhibition or genetic ablation of TRAF6 restored cell death levels in CGNs lacking RIP2. These results revealed an unexpected mechanism controlling CGN number and highlight how competitive interactions govern the logic of death receptor function.
The paper has just been published in Cell Reports.
Read the full paper HERE.
In this new paper, we show how the GDNF receptor GFRα1 functions cell-autonomously in subpopulations of olfactory bulb interneuron precursors to regulate their generation and allocation in the mammalian olfactory bulb.
GFRα1, a receptor for glial cell line-derived neurotrophic factor (GDNF), is critical for the development of the main olfactory system. The olfactory bulb (OB) of Gfra1 knockout mice showed significant reductions in the number of olfactory sensory neurons, mitral and tufted cells, as well as all major classes of OB GABAergic interneurons. However, the latter did not express significant levels of GFRα1, leaving the mechanism of action of GFRα1 in OB interneuron development unexplained. We have found that GFRα1 is highly expressed in the precursor cells that give rise to all major classes of OB interneurons, but is downregulated as these neurons mature. Conditional ablation of GFRα1 in embryonic GABAergic cells recapitulated the cell losses observed in global Gfra1 knockouts at birth. GFRα1 was also required for the sustained generation and allocation of OB interneurons in adulthood. Conditional loss of GFRα1 altered the migratory behaviour of neuroblasts along the rostral migratory stream (RMS) as well as RMS glial tunnel formation. Together, these data indicate that GFRα1 functions cell-autonomously in subpopulations of OB interneuron precursors to regulate their generation and allocation in the mammalian OB.
The paper has just been published in Biology Open.
Read the full paper HERE.
In this new paper, we show how the GDNF regulates survival of molecular layer interneurons in the cerebellum to control normal cerebellar motor learning. The paper has just been published in Cell Reports.
The role of neurotrophic factors as endogenous survival proteins for brain neurons remains contentious. In the cerebellum, the signals controlling survival of molecular layer interneurons (MLIs) are unknown, and direct evidence for the requirement of a full complement of MLIs for normal cerebellar function and motor learning has been lacking. Here, we show that Purkinje cells (PCs), the target of MLIs, express the neurotrophic factor GDNF during MLI development and survival of MLIs depends on GDNF receptors GFRα1 and RET. Conditional mutant mice lacking either receptor lose a quarter of their MLIs, resulting in compromised synaptic inhibition of PCs, increased PC firing frequency, and abnormal acquisition of eyeblink conditioning and vestibulo-ocular reflex performance, but not overall motor activity or coordination. These results identify an endogenous survival mechanism for MLIs and reveal the unexpected vulnerability and selective requirement of MLIs in the control of cerebellar-dependent motor learning.
Read the full paper HERE.
In this new paper, we show how the GFRα1 receptor regulates Purkinje cell migration independently of GDNF or RET, by limiting the function of NCAM. The paper has just been published in Cell Reports.
During embryonic development of the cerebellum, Purkinje cells (PCs) migrate away from the ventricular zone to form the PC plate. The mechanisms that regulate PC migration are incompletely understood. Here, we report that the neurotrophic receptor GFRα1 is transiently expressed in developing PCs and loss of GFRα1 delays PC migration. Neither GDNF nor RET, the canonical GFRα1 ligand and co-receptor, respectively, contribute to this process. Instead, we found that the neural cell adhesion molecule NCAM is co-expressed and directly interacts with GFRα1 in embryonic PCs. Genetic reduction of NCAM expression enhances wild-type PC migration and restores migration in Gfra1 mutants, indicating that NCAM restricts PC migration in the embryonic cerebellum. In vitro experiments indicated that GFRα1 can function both in cis and trans to counteract NCAM and promote PC migration. Collectively, our studies show that GFRα1 contributes to PC migration by limiting NCAM function.
Read the full paper HERE.
In our latest paper, we show how thalamo-cortical axons regulate the radial dispersion of neocortical GABAergic interneurons. The paper has just been published in eLife.
Neocortical GABAergic interneuron migration and thalamo-cortical axon (TCA) pathfinding follow similar trajectories and timing, suggesting they may be interdependent. The mechanisms that regulate the radial dispersion of neocortical interneurons are incompletely understood. In this new study we report that disruption of TCA innervation, or TCA-derived glutamate, affected the laminar distribution of GABAergic interneurons in mouse neocortex, resulting in abnormal accumulation in deep layers of interneurons that failed to switch from tangential to radial orientation. Expression of the KCC2 cotransporter was elevated in interneurons of denervated cortex, and KCC2 deletion restored normal interneuron lamination in the absence of TCAs. Disruption of interneuron NMDA receptors or pharmacological inhibition of calpain also led to increased KCC2 expression and defective radial dispersion of interneurons. Thus, although TCAs are not required to guide the tangential migration of GABAergic interneurons, they provide crucial signals that restrict interneuron KCC2 levels, allowing coordinated neocortical invasion of TCAs and interneurons.
Read the full paper HERE. (Supplemental information 31.6MB)
In our latest paper, we show how dimers of the p75NTR neurotrophin receptor are indipensable for p75NTR-mediated cell death in the central nervous system. The paper has just been published in the Journal of Neuroscience.
The oligomeric state and activation mechanism that enable p75 NTR to mediate these effects have recently been called into question. In this new study, we have investigated mutant mice lacking the p75NTR death domain (DD) or a highly conserved transmembrane (TM) cysteine residue (Cys 259) implicated in receptor dimerization and activation. Neuronal death induced by proneurotrophins or epileptic seizures was assessed and compared with responses in p75NTR knock-out mice and wild-type animals. Proneurotrophins induced apoptosis of cultured hippocampal and cortical neurons from wild-type mice, but mutant neurons lacking p75NTR, only the p75NTR DD, or just Cys259 were all equally resistant to proneurotrophin-induced neuronal death. Homo-FRET anisotropy experiments demonstrated that both NGF and proNGF induce conformational changes in p75 NTR that are dependent on the TM cysteine. In vivo, neuronal death induced by pilocarpine-mediated seizures was significantly reduced in the hippocampus and somatosensory, piriform, and entorhinal cortices of all three strains of p75 NTR mutant mice. Interestingly, the levels of protection observed in mice lacking the DD or only Cys 259 were identical to those of p75 NTR knock-out mice even though the Cys 259 mutant differed from the wild-type receptor in only one amino acid residue. We conclude that, both in vitro and in vivo, neuronal death induced by p75NTR requires the DD and TM Cys259, supporting the physiological relevance of DD signaling by disulfide-linked dimers of p75NTR in the CNS.
Read the full paper HERE.
A targeted effort to identify novel neurotrophic factors for midbrain dopaminergic neurons resulted in the isolation of GDNF (glial cell line-derived neurotrophic factor) from the supernatant of a rat glial cell line in 1993. Over two decades and 1200 papers later, the GDNF ligand family and their different receptor systems are now recognized as one of the major neurotrophic networks in the nervous system, important for the devel- opment, maintenance and function of a variety of neurons and glial cells. The many ways in which the four mem- bers of the GDNF ligand family can signal and function allow these factors to take part in the control of multiple types of processes, from neuronal survival to axon guidance and synapse formation in the developing nervous system, to synaptic function and regenerative responses in the adult. In this review, recently published in Neurobiology Of Disease, basic aspects of GDNF signaling mechanisms and receptor systems are first summarized followed by a review of current knowledge on the physiology of GDNF activities in the central nervous system, with an eye to its relevance for neurodegenerative and neuropsychiatric diseases. Read the full paper HERE.
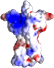
Our latest paper describes new NMR structures of the death domain in complex with downstream interactions RhoGDI and RIP2 as well as the death domain dimer. These are the first structural insights into p75NTR signaling and reveal many surprises for the death domain superfamily. The paper is now available online at eLife.
Death domains (DDs) mediate assembly of oligomeric complexes for activation of downstream signaling pathways through incompletely understood mechanisms. We report structures of complexes formed by the DD of p75 neurotrophin receptor (p75NTR) with RhoGDI, for activation of the RhoA pathway, with caspase recruitment domain (CARD) of RIP2 kinase, for activation of the NF-kB pathway, and with itself, revealing how DD dimerization controls access of intracellular effectors to the receptor. RIP2 CARD and RhoGDI bind to p75NTR DD at partially overlapping epitopes with over 100-fold difference in affinity, revealing the mechanism by which RIP2 recruitment displaces RhoGDI upon ligand binding. The p75NTR DD forms non-covalent, low-affinity symmetric dimers in solution. The dimer interface overlaps with RIP2 CARD but not RhoGDI binding sites, supporting a model of receptor activation triggered by separation of DDs. These structures reveal how competitive protein-protein interactions orchestrate the hierarchical activation of downstream pathways in non-catalytic receptors.
In our latest paper, we show that the p75 neurotrophin receptor p75NTR can signal very differently in diferent types of neurons. Using pharmacological and genetic techniques, we demonstrate that this is partly controlled by differential proteolytic cleavage of the receptor in different cell types. The new work has appeared online in the Journal of Cell Science.
Signaling by the p75 neurotrophin receptor (p75NTR) is often referred to as cell-context dependent, but neuron-type specific signaling by p75NTR has not been systematically investigated. Here, we report that p75NTR signals very differently in hippocampal neurons (HCNs) and cerebellar granule neurons (CGNs), and present evidence indicating that this is partly controlled by differential proteolytic cleavage. NGF induced caspase-3 activity and cell death in HCNs but not in CGNs, while it stimulated NFκB activity in CGNs but not in HCNs. HCNs and CGNs displayed different patterns of p75NTRproteolytic cleavage. While the p75NTR carboxy terminal fragment (CTF) was more abundant than the intracellular domain (ICD) in HCNs, CGNs exhibited fully processed ICD with very little CTF. Pharmacological or genetic blockade of p75NTR cleavage by gamma-secretase abolished NGF-induced upregulation of NFκB activity and enabled induction of CGN death, phenocopying the functional profile of HCNs. Thus, the activities of multifunctional receptors, such as p75NTR, can be tuned into narrower activity profiles by cell-type-specific differences in intracellular processes, such as proteolytic cleavage, leading to very different biological outcomes. Read the full article HERE.
In our latest paper, we demonstrate that spatially resolved RNA-seq is ideally suited for high resolution topographical mapping of genome-wide gene expression in heterogeneous anatomical structures such as the mammalian central nervous system. The work has appeared online in Genome Biology.
Cortical interneurons originating from the medial ganglionic eminence, MGE, are among the most diverse cells within the CNS. Different pools of proliferating progenitor cells are thought to exist in the ventricular zone of the MGE, but whether the underlying subventricular and mantle regions of the MGE are spatially patterned has not yet been addressed. In this work, we combined laser-capture microdissection and multiplex RNA-sequencing to map the transcriptome of MGE cells at a spatial resolution of 50 microns. Distinct groups of progenitor cells showing different stages of interneuron maturation were identified and topographically mapped based on their genome-wide transcriptional pattern. Although proliferating potential decreased rather abruptly outside the ventricular zone, a ventro-lateral gradient of increasing migratory capacity was identified, revealing heterogeneous cell populations within this neurogenic structure. Read the full article HERE.
In our latest paper, we report that the sensitivity of fat cells to signals that increase the breakdown of fat is linked to the receptor ALK7. The discovery, which is published in eLife, suggests that ALK7 is an interesting target for future strategies to treat obesity.
The ALK7 receptor is predominantly found in fat cells and tissues involved in controlling the metabolism. Intriguingly, mice with a mutation in ALK7 accumulate less fat than mice with a functional version of the protein. Until now, it has not been known why.
We created mice whose fat cells lack ALK7, but whose other cells all produce ALK7 as normal. We found that fat cells lacking the ALK7 receptor are more sensitive to adrenaline and noradrenaline signals, a finding that can explain why they accumulate less fat even though the mice were on a high-fat diet. Adrenaline and noradrenaline are central players in metabolism. These hormones trigger the burst of energy and increase in heart rate and blood pressure that are needed for the “fight-or-flight” response. The hormones normally stimulate the breakdown of fat, but when nutrients are plentiful, fat cells become resistant to this signal and instead store fat. This mechanism evolved to facilitate energy storage during times of abundant food supply, enhancing survival upon starvation. In the industrialized world where food is constantly accessible, this resistance can cause an unhealthy increase in body fat and result in obesity.
We then investigated if it is possible to prevent obesity by blocking ALK7. At present, there are no known ALK7 inhibitors, but we solved this by generating mice with a special mutation in ALK7 which renders it sensitive to inhibition by a chemical substance. This made it possible for us to block the receptor at any time in an otherwise normal adult animal.
– Using this approach, we could get these mice to be leaner on a high fat diet simply by administration of the chemical. This suggests that acute inhibition of the ALK7 receptor can prevent obesity in adult animals, says Tingqing Guo, first author of the study.
We have also showed that the ALK7 receptor works in a similar way in human fat cells as it does in mice.
– Overall, these results suggest that blockade of the ALK7 receptor could represent a novel strategy to combat human obesity, says Carlos Ibanez, principal investigator of the study.
The work was supported by grants from the European Research Council, Swedish Research Council, Strategic Research Program in Diabetes of Karolinska Institutet, Swedish Cancer Society, Knut and Alice Wallenberg Foundation, the National University of Singapore and the National Medical Research Council of Singapore. eLife is a peer-reviewed open-access scientific journal established at the end of 2012 by Nobel Prize Winner Randy Schekman, with support from the Howard Hughes Medical Institute, Max Planck Society and Wellcome Trust.
The paper is freely accessible and can be found HERE.
Diabetologia has now published online our latest paper describing differential actions of activins A and B and Smad proteins 2 and 3 on the regulation of insulin secretion by pancreatic beta cells (Wu et al., 2013).
Glucose-stimulated insulin secretion (GSIS) from pancreatic beta-cells is regulated by paracrine factors whose identity and mechanisms of action are incompletely understood. Activins are expressed in pancreatic islets and have been implicated in the regulation of GSIS. Activins A and B signal through a common set of intracellular components, but it is unclear whether they display similar or distinct functions in glucose homeostasis. Glucose homeostatic responses were examined in mice lacking activin B and in pancreatic islets derived from these mutants. The ability of activins A and B to regulate downstream signalling, ATP production and GSIS in islets and in beta-cells was compared. Mice lacking activin-B displayed elevated serum insulin levels and glucose-stimulated insulin release. Injection of a soluble activin B antagonist phenocopied these changes in wild type mice. Isolated pancreatic islets from mutant mice showed enhanced GSIS which could be rescued by exogenous activin B. Activin B negatively regulated GSIS and ATP production in wild type islets, while activin-A displayed opposite effects. The downstream mediator Smad3 responded preferentially to activin B in pancreatic islets and beta-cells, while Smad2 showed preference for activin A, indicating distinct signalling effects of the two activins. In line with this, overexpression of Smad3, but not Smad2, decreased GSIS in pancreatic islets. These results reveal a tug-of-war between activin ligands in the regulation of insulin secretion by beta-cells and suggest that manipulation of activin signalling could be a useful strategy for the control of glucose homeostasis in diabetes and metabolic disease.
Read the full article HERE.
Cold Spring Harbour Perspectives in Biology has published Carlos Ibanez’s review on the structure and physiology of the RET receptor tyrosine kinase as part of their collection of reviews on receptor tyrosine kinases. RET, GDNF family ligands, and GFRα coreceptors activate signaling pathways involved in kidney and nervous system development. RET mutations cause Hirschsprung’s disease and at least four cancers. Read the full paper HERE.
Cell Reports publishes today our latest paper describing a structure-function map of the death domain of the p75 neurotrophin receptor (Charalampopoulos et al. 2012)
Structural determinants underlying signaling specificity in the tumor necrosis factor receptor superfamily (TNFRSF) are poorly characterized and it is unclear whether different signaling outputs can be genetically dissociated. The p75 neurotrophin receptor (p75NTR), also known as TNFRSF16, is a key regulator of trophic and injury responses in the nervous system. In this paper, we describe a genetic approach to dissect p75NTR signaling and decipher its underlying logic. Structural determinants important for regulation of cell death, NF-kB and RhoA pathways were identified in the p75NTR death domain. Pro-apoptotic and pro-survival pathways mapped onto non-overlapping epitopes, demonstrating that different signaling outputs can be genetically separated in p75NTR. Dissociation of JNK and caspase-3 activities indicated that JNK is necessary but not sufficient for p75NTR-mediated cell death. RIP2 recruitment and RhoGDI release were mechanistically linked, indicating that competition for DD binding underlies cross-talk between NF-kB and RhoA pathways in p75NTR signaling. These results provide new insights into the logic of p75NTR signaling and pave the way for a genetic dissection of p75NTR function and physiology.
Read the full paper HERE.
The Journal of Neuroscience publishes today our paper on the role of the GDNF receptor GFRa1 in the main olfactory system (Marks et al. 2012). In this work, we investigated the consequences of GFRα1 deficiency for mouse olfactory system development and function.
GDNF and its receptor GFRα1 are prominently expressed in the olfactory epithelium (OE) and olfactory bulb (OB), but their importance for olfactory system development has been unknown. In the OE, we found that GFRα1 was expressed in basal precursors, immature olfactory sensory neurons (OSNs), and olfactory ensheathing cells (OECs), but was excluded from mature OSNs. The OE of newborn Gfra1 knock-out mice was thinner and contained fewer OSNs, but more dividing precursors, suggesting deficient neurogenesis. Immature OSN axon bundles were enlarged and associated OECs increased, indicating impaired migration of OECs and OSN axons. In the OB, GFRα1 was expressed in immature OSN axons and OECs of the nerve layer, as well as mitral and tufted cells, but was excluded from GABAergic interneurons. In newborn knock-outs, the nerve layer was dramatically reduced, exhibiting fewer axons and OECs. Bulbs were smaller and presented fewer and disorganized glomeruli and a significant reduction in mitral cells. Numbers of tyrosine hydroxylase-, calbindin-, and calretinin-expressing interneurons were also reduced in newborn mice lacking Gfra1. At birth, the OE and OB of Gdnf knock-out mice displayed comparable phenotypes. Similar deficits were also found in adult heterozygous Gfra1+/− mutants, which in addition displayed diminished responses in behavioral tests of olfactory function. We conclude that GFRα1 is critical for the development and function of the main olfactory system, contributing to the development and allocation of all major classes of neurons and glial cells.
Read the full paper HERE.
The FASEB Journal has published our paper on the role of the activin receptor ALK7 in the control of female reproduction (Sandoval-Guzman et al. 2012). In this work, we investigated the expression and function of the activin receptor ALK7 in the female reproductive axis using Alk7-knockout mice.
Alk7-knockout females showed delayed onset of puberty and abnormal estrous cyclicity, had abnormal diestrous levels of FSH and LH in serum, and their ovaries showed premature depletion of follicles, oocyte degeneration, and impaired responses to exogenous gonadotropins. In the arcuate nucleus, mutant mice showed reduced expression of Npy mRNA and lower numbers of Npy-expressing neurons than wild- type controls. Alk7 knockouts showed a selective loss of arcuate NPY/AgRP innervation in the medial preoptic area, a key central regulator of reproduction. These results indicate that ALK7 is an important regulator of female reproductive function and reveal a new role for activin signaling in the control of hypothalamic gene expression and wiring. Alk7 gene variants may contribute to female reproductive disorders in humans, such as polycystic ovary syndrome.
Read the full paper HERE.
Our review on p75 neurotrophin signaling in nervous system injury has been made available as a paper in press in the Trends In Neurosciences web site.
Injury or insult to the adult nervous system often results in reactivation of signaling pathways that are normally only active during development. The p75 neurotrophin receptor (p75NTR) is one such signaling molecule whose expression increases markedly following neural injury in many of the same cell types that express p75NTR during development. A series of studies during the past decade has demonstrated that p75NTR signaling contributes to neuronal and glial cell damage, axonal degeneration and dysfunction during injury and cellular stress. Why the nervous system reacts to injury by inducing a molecule that aids the demise of cells and axons is a biological paradox that remains to be explained satisfactorily. On the other hand, it may offer unique therapeutic opportunities for limiting the severity of nervous system injury and disease.
Read the full paper HERE.
The Journal of Cell Science publishes today our paper on the interaction between MET and GDNF signaling in the control of cortical GABAergic interneuron development (Perrinjaquet et al. 2011). This work demonstrates that responsiveness to GDNF in Gfra1 knock-out GABAergic interneurons can be restored upon addition of soluble GFRa1. As these neurons express neither RET nor NCAM, this result is only compatible with the existence of a novel transmembrane receptor partner for the GDNF-GFRa1 complex in GABAergic interneurons. Neither ErbB4 nor MET were found to fullfil this role. Unexpectedly, however, inhibition of MET (or its ligand HGF) per se promoted neuronal differentiation and migration and enhanced the activity of GDNF on GABAergic neurons. In agreement with this, Met mutant neurons showed enhanced responsiveness to GDNF and elevated levels of GFRa1 expression, both in vitro and in vivo. These results demonstrate the existence of a novel transmembrane receptor partner for the GDNF–GFRa1 complex and uncover an unexpected interplay between GDNF–GFRa1 and HGF–MET signaling in the early diversification of cortical GABAergic interneuron subtypes. Read the full paper HERE.
|
|